WHO approves first mpox testing kit ‘Alinity m MPXV assay’ developed by Abbott Molecular Inc.
The first test for diagnosing mpox has been approved by the World Health Organisation (WHO) under its Emergency Use Listing (EUL). Mpox, categorized as a public health emergency, is an illness caused by the monkeypox virus that results in flu-like symptoms along with a rash and blisters on the skin.
Abbott Molecular Inc. developed the Alinity m MPXV assay for in vitro diagnostic (IVD) testing, also known as mpox testing.

The test has received approval for emergency usage, signifying an important advancement in enhancing testing capabilities in nations combatting mpox epidemics.
Early detection of mpox is crucial for its prompt treating
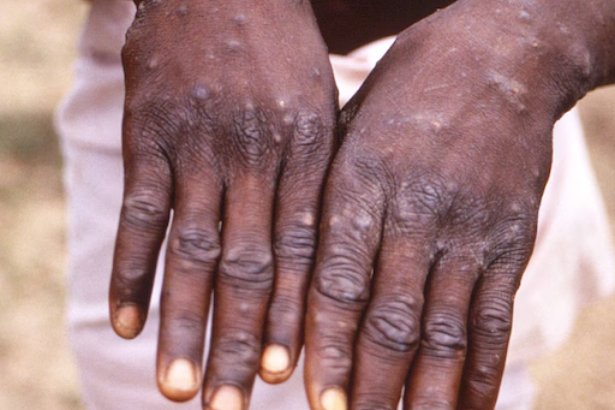
The demand for rapid and precise mpox testing has increased, particularly in areas such as Africa, where insufficient testing has played a role in the continuous transmission of the virus, according to the official report from the WHO.
Detecting mpox early is crucial for promptly treating it, handling outbreaks, and containing its transmission.
Also read: Centre confirms first case of mpox; says ‘no risk to public’
More than 30,000 suspected mpox cases were detected in Africa in 2024
In Africa, more than 30,000 suspected mpox cases were documented in 2024, with the Democratic Republic of the Congo, Burundi, and Nigeria having the most cases.
Only 37% of possible cases in the Democratic Republic of the Congo have undergone testing this year, underscoring the need for improved testing methods.
Process of mpox diagnostic testing:
- According to WHO guidelines, Mpox is verified using nucleic acid amplification testing (NAAT).
- The most reliable samples for diagnosis come from skin lesions.
- Through the examination of skin rash samples, medical professionals are able to efficiently verify suspected mpox cases, facilitating a faster response and provision of treatment.
‘This is a major achievement in increasing testing in impacted nations’: Dr Yukiko Nakatani
“This is a significant milestone in expanding testing in affected countries. Increasing access to quality testing is central to helping countries contain the virus and protect their populations, especially in underserved areas,” said Dr Yukiko Nakatani, WHO Assistant Director-General for Access to Medicines and Health Products.